We have all heard politicians, time after time again, claiming they need more research before they could even entertain the idea of changing their stance on adult-use of cannabis. Why is it that we need to spend so much time debating something we all know was based on false accusations, claims and flat out lies from a time when it was “OK” for leadership to make racist associations. Don’t believe us, educate yourself on Harry Anslinger.

America’s War on Drugs
We are suppose to be living in a time of unlimited information, yet we continue to put people in positions of leadership who cannot seem to be more lackadaisical when it comes to educating themselves. We are better than this and we should hold people accountable.
Adult-use is a no brainer. We have an opportunity to correct the wrongs of the past, provide economic and communal opportunities all the while generating new income streams for the State of Connecticut. The information is available and it is here for all to share. Keep big cannabis companies out of our state and give residents the first shot to work for Connecticut’s new industry. No one will ever want more for Connecticut, than someone from Connecticut.
Clinical Studies
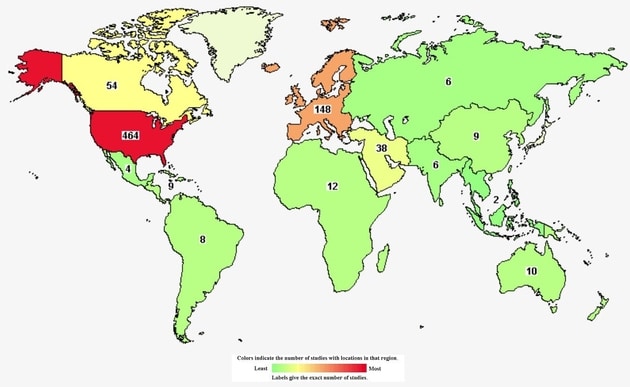
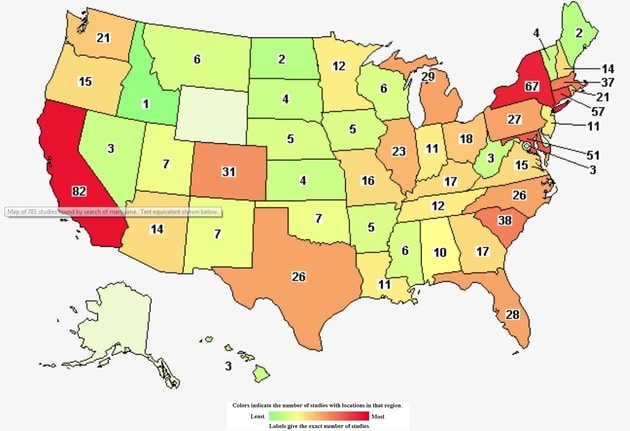
Did you know that there are 781 cannabis-related clinical studies in the world? Did you know that 464 are hosted in the US? Better yet 57 are in Connecticut. What is more questionable is the type of clinical studies being conducted. What is their end game? You decide.
For any veterans reading this… take a look at the studies currently ongoing at the Veteran Affairs Hospital right in West Haven…
Study List:
Study 1:
Title: Examine the Feasibility of a Standardized Field Test for Marijuana Impairment: Laboratory Evaluations
Status: Recruiting
Study Results: No Results Available
Conditions: Marijuana Impairment
Interventions: Drug: Low Dose THC Marijuana|Drug: High Dose THC Marijuana|Drug: Placebo Marijuana
Locations: Olin Neuropsychiatry Research Center, Hartford, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT03191084
Study 2:
Title: Neuroscience of Marijuana Impaired Driving
Status: Recruiting
Study Results: No Results Available
Conditions: Marijuana Impairment
Interventions: Drug: Low dose THC marijuana|Drug: High dose THC marijuana|Drug: Placebo marijuana
Locations: Olin Neuropsychiatry Research Center, Hartford, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT02757313
Study 3:
Title: Neuroscience of Alcohol and Marijuana Impaired Driving
Status: Recruiting
Study Results: No Results Available
Conditions: Marijuana Usage|Alcohol Drinking
Interventions: Drug: One day with a dose of placebo cannabis paired with alcohol|Drug: One day with a dose of active THC cannabis paired with alcohol
Locations: Olin Neuropsychiatry Research Center, Institute of Living, Hartford Hospital, Hartford, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT03431987
Study 4:
Title: Marijuana Treatment Project 4
Status: Active, not recruiting
Study Results: No Results Available
Conditions: Marijuana Dependence
Interventions: Behavioral: Contingency Management|Behavioral: Individualized Assessment & Treatment|Behavioral: Cognitive-Behavioral Treatment
Locations: University of Connecticut Health Center, Farmington, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT02030665
Study 5:
Title: Marijuana in Combination With Opioids in Palliative and Hospice Patients
Status: Enrolling by invitation
Study Results: No Results Available
Conditions: Pain Management in Terminally Ill Patients Receiving Scheduled Opioid Therapy
Interventions: Drug: Medical Marijuana
Locations: The Connecticut Hospice Inc., Branford, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT03233633
Study 6:
Title: Marijuana Treatment Project – 3
Status: Completed
Study Results: No Results Available
Conditions: Marijuana Dependence
Interventions: Behavioral: Reinforcement for homework completion|Behavioral: Reinforcement for Abstinence|Behavioral: Case Management
Locations: University of Connecticut Health Center, Farmington, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT00107588
Study 7:
Title: Effectiveness of Selegiline in Treating Marijuana Dependent Individuals – 1
Status: Completed
Study Results: No Results Available
Conditions: Marijuana Abuse
Interventions: Drug: Selegiline hydrochloride|Drug: Placebo
Locations: MRU, New Haven, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT00218517
Study 8:
Title: The Neural Correlates of Cannabis Use
Status: Recruiting
Study Results: No Results Available
Conditions: Cannabis Dependence|Healthy
Interventions: Other: [11-C]OMAR
Locations: Conneticut Mental Health Center, New Haven, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT03104257
Study 9:
Title: Brief Interventions With Text Messaging to Reduce Adolescent Alcohol and Marijuana Use
Status: Completed
Study Results: No Results Available
Conditions: Alcohol Drinking|Marijuana
Interventions: Behavioral: Text Messaging and Brief Negotiation Interview
Locations: Yale New Haven Hospital, New Haven, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT03401333
Study 10:
Title: Safety and Efficacy of a FAAH-Inhibitor to Treat Cannabis Withdrawal
Status: Active, not recruiting
Study Results: No Results Available
Conditions: Cannabis Dependence
Interventions: Drug: PF-04457845|Drug: Placebo
Locations: Yale University School of Medicine, New Haven, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT01618656
Study 11:
Title: Galantamine Effects on Cognitive Function in Marijuana Users
Status: Completed
Study Results: No Results Available
Conditions: Memory
Interventions: Drug: Placebo|Drug: Galantamine
Locations: Department of Veterans Affairs, West Haven, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT00969696
Study 12:
Title: Adaptive Treatment for Adolescent Cannabis Use Disorders
Status: Completed
Study Results: No Results Available
Conditions: Mental Disorders|Addictive Behaviors|Cannabis Use Disorder
Interventions: Behavioral: ACRA|Behavioral: CBT
Locations: University of Connecticut Health Center, Farmington, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT01656707
Study 13:
Title: Imaging the Neurochemistry of Drug Addiction With PET
Status: Recruiting
Study Results: No Results Available
Conditions: Smoking|Cannabis
Interventions: Drug: cannabis
Locations: Connecticut Mental Health Center, New Haven, Connecticut, United States|Yale Magnetic Resonance Research Center, New Haven, Connecticut, United States|Yale PET Center, New Haven, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT02817698
Study 14:
Title: Dronabinol Interactions With Cognitive Enhancing Drug in Humans
Status: Completed
Study Results: Has Results
Conditions: Cannabis|Marijuana Abuse
Interventions: Drug: drug condition
Locations: Department of Veterans Affairs Hospital, West Haven, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT00842985
Study 15:
Title: Pharmacogenetics of Cannabinoid Response
Status: Active, not recruiting
Study Results: No Results Available
Conditions: COMT Gene Polymorphism
Interventions: Drug: delta 9 tetrahydrocannabinol
Locations: VA Connecticut Healthcare System, West Haven, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT00678730
Study 16:
Title: Maximizing the Efficacy of Cognitive Behavior Therapy and Contingency Management
Status: Completed
Study Results: No Results Available
Conditions: Marijuana Dependence
Interventions: Behavioral: Standard CBT|Behavioral: CBT+CM/adherence|Behavioral: CM/abstinence|Behavioral: CM/abstinence+CBT
Locations: ASAP/1 Long Wharf, New Haven, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT00350649
Study 17:
Title: Cannabinoids, Neural Synchrony, and Information Processing
Status: Active, not recruiting
Study Results: No Results Available
Conditions: Cannabis|Psychotic Disorders
Interventions: Drug: THC|Drug: Placebo
Locations: VA Connecticut Healthcare System, West Haven, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT00708994
Study 18:
Title: Development of a Videogame Prototype Targeting Cigarette and Marijuana Smoking, and Tobacco Product Prevention Among Young Adolescents (smokeScreen)
Status: Completed
Study Results: No Results Available
Conditions: Smoking
Interventions: Device: smokeSCREEN video game
Locations: Hamden Youth Center, Hamden, Connecticut, United States|Leadership, Education, and Athletics Partnership (LEAP) Inc., New Haven, Connecticut, United States|Farnam Neighborhood House, New Haven, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT03722888
Study 19:
Title: Imaging Cannabinoid Receptors Using Positron Emission Tomography (PET) Scanning
Status: Recruiting
Study Results: No Results Available
Conditions: Schizophrenia|Cannabis Dependence|Prodromal for Psychotic Illness|Family History of Alcoholism|Healthy Control
Interventions: Radiation: [11-C]OMAR
Locations: Connecticut Mental Health Center, Clinical Neuroscience Research Unit, New Haven, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT01730781
Study 20:
Title: The Effects of ∆-9-THC and Naloxone in Humans
Status: Active, not recruiting
Study Results: No Results Available
Conditions: Healthy
Interventions: Drug: Naloxone|Drug: Delta-9-THC|Drug: Placebo
Locations: VA Connecticut Healthcare System, West Haven, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT01591629
Study 21:
Title: Therapeutic Substance Abuse Treatment in Pregnancy – 1
Status: Completed
Study Results: Has Results
Conditions: Alcohol Abuse|Cocaine Abuse|Marijuana Abuse
Interventions: Behavioral: MI-CBT|Behavioral: Brief Advice
Locations: Bridgeport Hospital, Bridgeport, Connecticut, United States|Yale-New Haven Hospital, New Haven, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT00227903
Study 22:
Title: Cannabinoids and Cerebellar-Motor Functioning
Status: Unknown status
Study Results: No Results Available
Conditions: Cannabis|Psychotic Disorders
Interventions: Drug: THC|Drug: Placebo
Locations: VA Connecticut Healthcare System, West Haven, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT01853020
Study 23:
Title: Effects of Delta-9-THC and Iomazenil in Healthy Humans
Status: Active, not recruiting
Study Results: No Results Available
Conditions: Schizophrenia|Mental Disorders|Psychotic Disorders
Interventions: Drug: THC and Iomazenil|Drug: Placebo (control)
Locations: VA Connecticut Healthcare System, West Haven, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT00982982
Study 24:
Title: Development of a Videogame Prototype Targeting Cigarette and Marijuana Smoking, and Tobacco Product Prevention Among Young Adolescents
Status: Completed
Study Results: No Results Available
Conditions: Smoking
Interventions: Behavioral: smokeSCREEN game
Locations: Hamden Youth Center, Hamden, Connecticut, United States|Leadership, Education, and Athletics Partnership (LEAP) Inc., New Haven, Connecticut, United States|Yale New Haven Hospital, New Haven, Connecticut, United States|Farnam Neighborhood House, New Haven, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT03107936
Study 25:
Title: Ethanol and Cannabinoid Effects on Simulated Driving and Related Cognition: Sub-Study II
Status: Active, not recruiting
Study Results: No Results Available
Conditions: Cannabis|Alcohol Effect|Driving Under the Influence of Alcohol and Other Drugs
Interventions: Drug: Active inhaled cannabis|Drug: Placebo|Drug: Active Oral Ethanol
Locations: Biological Studies Unit, VA Connecticut Healthcare System, West Haven, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT02710097
Study 26:
Title: Treatment for Teens With Alcohol Abuse and Depression
Status: Active, not recruiting
Study Results: No Results Available
Conditions: AOD Use, Abuse, and Dependence|Depression
Interventions: Behavioral: MET/CBT-12|Behavioral: CBT-D|Other: D-TAU
Locations: University of Connecticut Health Center, Farmington, Connecticut, United States|Duke Child and Family Study Center, Durham, North Carolina, United States
URL: https://ClinicalTrials.gov/show/NCT02227589
Study 27:
Title: The Effects of Cannabidiol and ∆-9-THC in Humans
Status: Active, not recruiting
Study Results: No Results Available
Conditions: Healthy
Interventions: Drug: Cannabidiol|Drug: Delta-9-THC|Drug: Placebo
Locations: VA Connecticut Healthcare System, West Haven, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT01180374
Study 28:
Title: Gender Related Differences in the Acute Effects of Delta-9-Tetrahydrocannabinol in Healthy Humans: Sub-Study I
Status: Recruiting
Study Results: No Results Available
Conditions: Cannabis
Interventions: Drug: Placebo|Drug: Dronabinol
Locations: Biological Studies Unit at the VA Connecticut Healthcare System, Yale School of Medicine, West Haven, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT02811510
Study 29:
Title: Gender Related Differences in the Acute Effects of Delta-9-Tetrahydrocannabinol in Healthy Humans
Status: Recruiting
Study Results: No Results Available
Conditions: Cannabis
Interventions: Drug: THC|Drug: Placebo
Locations: Biological Studies Unit at the VA Connecticut Healthcare System, Yale School of Medicine, West Haven, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT02781519
Study 30:
Title: Ethanol and Cannabinoid Effects on Simulated Driving and Related Cognition: Sub-Study I
Status: Recruiting
Study Results: No Results Available
Conditions: Cannabis|Alcohol Effect|Driving Under the Influence of Alcohol and Other Drugs
Interventions: Drug: Active inhaled delta-9-THC|Drug: Placebo|Drug: Active Oral Ethanol
Locations: Biological Studies Unit, VA Connecticut Healthcare System, West Haven, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT02709954
Study 31:
Title: Ethanol and Cannabinoid Effects on Simulated Driving and Related Cognition
Status: Recruiting
Study Results: No Results Available
Conditions: Cannabis|Alcohol Effect|Driving Under the Influence of Alcohol and Other Drugs
Interventions: Drug: Active delta-9-THC|Drug: Placebo|Drug: Active Ethanol
Locations: Biological Studies Unit, VA Connecticut Healthcare System, West Haven, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT02404688
Study 32:
Title: Cannabinoids, Learning, and Memory
Status: Recruiting
Study Results: No Results Available
Conditions: Cannabis|Psychotic Disorders
Interventions: Drug: THC|Drug: Placebo
Locations: VA Connecticut Healthcare System, West Haven, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT02407808
Study 33:
Title: Ethanol and Cannabinoid Effects on Simulated Driving and Related Cognition: Substudy III
Status: Recruiting
Study Results: No Results Available
Conditions: Cannabis|Alcohol Effect|Driving Under the Influence of Alcohol and Other Drugs
Interventions: Drug: Active Dronabinol|Drug: Placebo|Drug: Active Ethanol
Locations: Biological Studies Unit, VA Connecticut Healthcare System, West Haven, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT02710331
Study 34:
Title: Probing the Cannabinoid System in Individuals With a Family History of Psychosis
Status: Recruiting
Study Results: No Results Available
Conditions: Psychosis|Cannabis Use|THC|Marijuana|Schizophrenia
Interventions: Drug: Placebo|Drug: Very Low Dose THC|Drug: Low Dose THC
Locations: VA Connecticut Healthcare System, West Haven, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT02102113
Study 35:
Title: Cannabinoid Receptor Function & Alcoholism
Status: Active, not recruiting
Study Results: No Results Available
Conditions: Alcoholism
Interventions: Drug: THC|Drug: Placebo
Locations: VA Connecticut Healthcare System, West Haven, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT00624715
Study 36:
Title: Screening to Augment Referral to Treatment- Project START
Status: Completed
Study Results: Has Results
Conditions: Alcohol Abuse|Tobacco Use Disorder|Marijuana Abuse|Substance-Related Disorders
Interventions: Behavioral: Motivational Interview|Other: Treatment as Usual
Locations: Yale-New Haven Hospital- York St Campus, New Haven, Connecticut, United States|Yale-New Haven Hospital-Chapel St Campus, New Haven, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT01539525
Study 37:
Title: Contingency Management for Smoking in Substance Abusers
Status: Completed
Study Results: No Results Available
Conditions: Cigarette Smoking
Interventions: Behavioral: Contingency Management|Behavioral: Brief smoking cessation counseling
Locations: University of Connecticut Health Center, Farmington, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT00683033
Study 38:
Title: Healthy Activities for Prize Incentives
Status: Completed
Study Results: No Results Available
Conditions: Substance Abuse|HIV Infections
Interventions: Behavioral: contingency management
Locations: Connections, Inc., Hartford, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT00717444
Study 39:
Title: CB1R in Synthetic Psychoactive Cannabinoids
Status: Recruiting
Study Results: No Results Available
Conditions: Drug Dependence
Interventions: Other: [11-C]OMAR
Locations: Connecticut Mental Health Center, New Haven, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT03539575
Study 40:
Title: Cognitive and Psychophysiological Effects of Delta-9-Tetrahydrocannabinol in Bipolar Disorder
Status: Active, not recruiting
Study Results: No Results Available
Conditions: Delta-9-Tetrahydroncannabinol|Bipolar Disorder|Healthy Controls
Interventions: Drug: 4 mg Delta-9-THC|Drug: Placebo|Drug: 2 mg Delta-9-THC
Locations: Biological Studies Unit, VA Connecticut Healthcare System, West Haven, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT03206463
Study 41:
Title: N-acetylcysteine Effects on Tetrahydrocannabinol
Status: Active, not recruiting
Study Results: No Results Available
Conditions: Healthy
Interventions: Drug: Delta-9-THC|Drug: N-acetylcysteine|Drug: Placebo
Locations: VA Connecticut Healthcare System, West Haven, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT02335060
Study 42:
Title: Achieving Cannabis Cessation-Evaluating N-Acetylcysteine Treatment
Status: Completed
Study Results: Has Results
Conditions: Cannabis Dependence
Interventions: Drug: N-Acetylcysteine|Drug: Placebo
Locations: UCLA Integrated Substance Abuse Programs, Los Angeles, California, United States|APT Foundation, Inc., New Haven, Connecticut, United States|University of Kentucky, Lexington, Kentucky, United States|CODA, Inc., Portland, Oregon, United States|Behavioral Health Services of Pickens County, Pickens, South Carolina, United States|University of Texas Health Science Center at San Antonio, San Antonio, Texas, United States
URL: https://ClinicalTrials.gov/show/NCT01675661
Study 43:
Title: Testing the Interactive Effects of Delta-9-Tetrahydrocannabinol and Pregnenolone
Status: Recruiting
Study Results: No Results Available
Conditions: Healthy
Interventions: Drug: Active Delta-9-THC|Drug: Active Pregnenolone|Drug: Placebo
Locations: Biological Studies Unit at the VA Connecticut Healthcare System, Yale School of Medicine, West Haven, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT02576912
Study 44:
Title: Cannabidiol Treatment in Patients With Early Psychosis
Status: Active, not recruiting
Study Results: No Results Available
Conditions: Schizophrenia|Schizoaffective Disorder
Interventions: Drug: Cannabidiol|Drug: Placebo
Locations: Connecticut Mental Health Center, New Haven, Connecticut, United States|VA Connecticut Healthcare System, West Haven, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT02504151
Study 45:
Title: Cannabinoid Modulation of Pain
Status: Active, not recruiting
Study Results: No Results Available
Conditions: Pain
Interventions: Other: Thermal|Device: Electrical|Other: Capsaicin
Locations: VA Connecticut Healthcare System, West Haven, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT01595620
Study 46:
Title: Testing the Interactive Effects of Delta-9-Tetrahydrocannabinol and Pregnenolone: Sub-Study I
Status: Recruiting
Study Results: No Results Available
Conditions: Healthy
Interventions: Drug: Active Dronabinol|Drug: Active Pregnenolone|Drug: Placebo Dronabinol|Drug: Placebo Pregnenolone
Locations: Biological Studies Unit at the VA Connecticut Healthcare System, Yale School of Medicine, West Haven, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT02811939
Study 47:
Title: A Naturalistic Study of Adolescents and Young Adults in Treatment for Opioid Dependence
Status: Completed
Study Results: No Results Available
Conditions: Opioid Dependence
Interventions: Other: Naturalistic Prospective Cohort
Locations: APT Foundation, New Haven, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT02718352
Study 48:
Title: An Interactive Game for HIV Prevention in Early Adolescents
Status: Completed
Study Results: No Results Available
Conditions: HIV|Risk Reduction
Interventions: Behavioral: Experimental Video Game|Behavioral: Off the Shelf Video Game
Locations: Yale School of Medicine, New Haven, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT01666496
Study 49:
Title: Stage I Randomized Trial of Mentalization-Based Therapy for Substance Using Mothers of Infants and Toddlers
Status: Completed
Study Results: Has Results
Conditions: Maternal Substance Use|Child Abuse and Neglect
Interventions: Behavioral: Mentalizing Therapy for Substance Using Mothers|Behavioral: Standard Parent Education for Substance Using Mothers
Locations: The APT Foundation, New Haven, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT00319436
Study 50:
Title: Secondary HIV Prevention and Adherence Among HIV-infected Drug Users
Status: Completed
Study Results: No Results Available
Conditions: Risk Behavior|Medication Adherence|HIV
Interventions: Behavioral: 3H+ (Holistic Health for HIV)|Behavioral: HHRP+ (Holistic Health Recovery Program)
Locations: APT Foundation, New Haven, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT01741311
Study 51:
Title: Study of of APL-2 Therapy in Patients Geographic Atrophy
Status: Completed
Study Results: No Results Available
Conditions: Geographic Atrophy
Interventions: Drug: APL-2|Other: Sham Procedure
Locations: Retinal Research Institute, Phoenix, Arizona, United States|Retina Vitreous Asociates Mdical Goup, Beverly Hills, California, United States|The Gavin Herbert Eye Institute/UC Irvine, Irvine, California, United States|Byers Eye Institute at Standford, Stanford School of Medicine, Palo Alto, California, United States|New England Retina Associates, New London, Connecticut, United States|Florida Eye Microsurgical Institute, Inc., Boynton Beach, Florida, United States|Retina Health Center, Fort Myers, Florida, United States|Bascom Palmer Eye Institute, Miami, Florida, United States|South East Retina, Augusta, Georgia, United States|Midwest Eye Institute, Indianapolis, Indiana, United States|Ophthalmic Consultants of Boston, Boston, Massachusetts, United States|Associated Retinal Consultants PC, Grand Rapids, Michigan, United States|Associated Retinal Consultants, PC, Traverse City, Michigan, United States|Eyesight Opthalmic Services PA, Portsmouth, New Hampshire, United States|Vitreous Retina Macula Consultants of New York, New York, New York, United States|Charlotte Eye Ear Nose and Throat Associates, Charlotte, North Carolina, United States|Duke University, Duke Eye Center, Durham, North Carolina, United States|Retina Associates of Cleveland, Cleveland, Ohio, United States|Cleveland Clinica Foundation/ Cole Eye Institute, Cleveland, Ohio, United States|Tennessee Retina, PC, Nashville, Tennessee, United States|Retina Research Institute of Texas, Abilene, Texas, United States|Retina Research Center, Austin, Texas, United States|Retina Consultants of Houston, Houston, Texas, United States|Valley Retina Institute, PA, McAllen, Texas, United States|Retina Consultants of Houston (The Woodlands), The Woodlands, Texas, United States|Marsden Eye Specialists, Paramatta, New South Wales, Australia|Save Sight Institute, Sydney Eye Hospital, Sydney, New South Wales, Australia|Sydney Retina Clinic and Day Surgery, Sydney, New South Wales, Australia|Sydney West Retina, Westmead, New South Wales, Australia|Hobart eye Surgeons, Hobart, Tasmania, Australia|Tasmanian Eye Institute, South Launceston, Tasmania, Australia|Royal Victorian Eye and Ear Hospital, East Melbourne, Victoria, Australia|Lions Eye Institute, Nedlands, Western Australia, Australia|Auckland Eye, Remuera, Auckland, New Zealand|Southern Eye Specialists, Merivale, Christchurch, New Zealand
URL: https://ClinicalTrials.gov/show/NCT02503332
Study 52:
Title: National Adaptive Trial for PTSD Related Insomnia
Status: Not yet recruiting
Study Results: No Results Available
Conditions: Insomnia
Interventions: Drug: Trazodone|Drug: Eszopiclone|Drug: Gabapentin|Other: Placebo
Locations: VA Connecticut Healthcare System West Haven Campus, West Haven, CT, West Haven, Connecticut, United States
URL: https://ClinicalTrials.gov/show/NCT03668041
Study 53:
Title: Eltrombopag To Initiate And Maintain Interferon Antiviral Treatment To Benefit Subjects With Hepatitis C Liver Disease
Status: Completed
Study Results: Has Results
Conditions: Hepatitis C, Chronic
Interventions: Drug: eltrombopag|Drug: placebo
Locations: GSK Investigational Site, Birmingham, Alabama, United States|GSK Investigational Site, Tucson, Arizona, United States|GSK Investigational Site, Anaheim, California, United States|GSK Investigational Site, Arcadia, California, United States|GSK Investigational Site, La Jolla, California, United States|GSK Investigational Site, Los Angeles, California, United States|GSK Investigational Site, Los Angeles, California, United States|GSK Investigational Site, Lynwood, California, United States|GSK Investigational Site, Sacramento, California, United States|GSK Investigational Site, San Clemente, California, United States|GSK Investigational Site, San Diego, California, United States|GSK Investigational Site, San Mateo, California, United States|GSK Investigational Site, Aurora, Colorado, United States|GSK Investigational Site, New Haven, Connecticut, United States|GSK Investigational Site, Washington, District of Columbia, United States|GSK Investigational Site, Washington, District of Columbia, United States|GSK Investigational Site, Bradenton, Florida, United States|GSK Investigational Site, Jacksonville, Florida, United States|GSK Investigational Site, Miami, Florida, United States|GSK Investigational Site, Honolulu, Hawaii, United States|GSK Investigational Site, Chicago, Illinois, United States|GSK Investigational Site, Indianapolis, Indiana, United States|GSK Investigational Site, Lexington, Kentucky, United States|GSK Investigational Site, New Orleans, Louisiana, United States|GSK Investigational Site, Boston, Massachusetts, United States|GSK Investigational Site, Burlington, Massachusetts, United States|GSK Investigational Site, Worcester, Massachusetts, United States|GSK Investigational Site, Detroit, Michigan, United States|GSK Investigational Site, Detroit, Michigan, United States|GSK Investigational Site, Jackson, Mississippi, United States|GSK Investigational Site, St. Louis, Missouri, United States|GSK Investigational Site, St. Louis, Missouri, United States|GSK Investigational Site, Lebanon, New Hampshire, United States|GSK Investigational Site, Bronx, New York, United States|GSK Investigational Site, Manhasset, New York, United States|GSK Investigational Site, New York, New York, United States|GSK Investigational Site, New York, New York, United States|GSK Investigational Site, Rochester, New York, United States|GSK Investigational Site, Syracuse, New York, United States|GSK Investigational Site, Asheville, North Carolina, United States|GSK Investigational Site, Durham, North Carolina, United States|GSK Investigational Site, Kinston, North Carolina, United States|GSK Investigational Site, Morganton, North Carolina, United States|GSK Investigational Site, Tulsa, Oklahoma, United States|GSK Investigational Site, Portland, Oregon, United States|GSK Investigational Site, Hershey, Pennsylvania, United States|GSK Investigational Site, Philadelphia, Pennsylvania, United States|GSK Investigational Site, Germantown, Tennessee, United States|GSK Investigational Site, Mountain Home, Tennessee, United States|GSK Investigational Site, Nashville, Tennessee, United States|GSK Investigational Site, Nashville, Tennessee, United States|GSK Investigational Site, Nashville, Tennessee, United States|GSK Investigational Site, Dallas, Texas, United States|GSK Investigational Site, Galveston, Texas, United States|GSK Investigational Site, Houston, Texas, United States|GSK Investigational Site, San Antonio, Texas, United States|GSK Investigational Site, Charlottesville, Virginia, United States|GSK Investigational Site, Richmond, Virginia, United States|GSK Investigational Site, Seattle, Washington, United States|GSK Investigational Site, Garran, Australian Capital Territory, Australia|GSK Investigational Site, Camperdown, New South Wales, Australia|GSK Investigational Site, Randwick, New South Wales, Australia|GSK Investigational Site, Cairns, Queensland, Australia|GSK Investigational Site, Herston, Queensland, Australia|GSK Investigational Site, Clayton, Victoria, Australia|GSK Investigational Site, Fitzroy, Victoria, Australia|GSK Investigational Site, Parkville, Victoria, Australia|GSK Investigational Site, Prahran, Victoria, Australia|GSK Investigational Site, Nedlands, Western Australia, Australia|GSK Investigational Site, Bruxelles, Belgium|GSK Investigational Site, Edegem, Belgium|GSK Investigational Site, Gent, Belgium|GSK Investigational Site, Leuven, Belgium|GSK Investigational Site, Campinas, São Paulo, Brazil|GSK Investigational Site, São Paulo, Brazil|GSK Investigational Site, Edmonton, Alberta, Canada|GSK Investigational Site, Victoria, British Columbia, Canada|GSK Investigational Site, Winnipeg, Manitoba, Canada|GSK Investigational Site, Barrie, Ontario, Canada|GSK Investigational Site, Hamilton, Ontario, Canada|GSK Investigational Site, Hamilton, Ontario, Canada|GSK Investigational Site, London, Ontario, Canada|GSK Investigational Site, Ottawa, Ontario, Canada|GSK Investigational Site, Toronto, Ontario, Canada|GSK Investigational Site, Toronto, Ontario, Canada|GSK Investigational Site, Toronto, Ontario, Canada|GSK Investigational Site, Toronto, Ontario, Canada|GSK Investigational Site, Montreal, Quebec, Canada|GSK Investigational Site, Montreal, Quebec, Canada|GSK Investigational Site, Hradec Kralove, Czech Republic|GSK Investigational Site, Olomouc, Czech Republic|GSK Investigational Site, Praha 6, Czech Republic|GSK Investigational Site, Cairo, Egypt|GSK Investigational Site, Mansoura, Egypt|GSK Investigational Site, Shebeen El-Kom, Menoufeya, Egypt|GSK Investigational Site, Besançon, France|GSK Investigational Site, Bondy, France|GSK Investigational Site, Dijon cedex, France|GSK Investigational Site, Grenoble, France|GSK Investigational Site, Lille, France|GSK Investigational Site, Marseille Cedex 08, France|GSK Investigational Site, Orléans, France|GSK Investigational Site, Paris Cedex 12, France|GSK Investigational Site, Paris Cedex 13, France|GSK Investigational Site, Rouen, France|GSK Investigational Site, Strasbourg, France|GSK Investigational Site, Vandoeuvre Les Nancy, France|GSK Investigational Site, Freiburg, Baden-Wuerttemberg, Germany|GSK Investigational Site, Heidelberg, Baden-Wuerttemberg, Germany|GSK Investigational Site, Mannheim, Baden-Wuerttemberg, Germany|GSK Investigational Site, Stuttgart, Baden-Wuerttemberg, Germany|GSK Investigational Site, Ulm, Baden-Wuerttemberg, Germany|GSK Investigational Site, Deggendorf, Bayern, Germany|GSK Investigational Site, Hof/Saale, Bayern, Germany|GSK Investigational Site, Muenchen, Bayern, Germany|GSK Investigational Site, Muenchen, Bayern, Germany|GSK Investigational Site, Regensburg, Bayern, Germany|GSK Investigational Site, Wuerzburg, Bayern, Germany|GSK Investigational Site, Beeskow, Brandenburg, Germany|GSK Investigational Site, Frankfurt, Hessen, Germany|GSK Investigational Site, Frankfurt, Hessen, Germany|GSK Investigational Site, Kassel, Hessen, Germany|GSK Investigational Site, Goettingen, Niedersachsen, Germany|GSK Investigational Site, Hannover, Niedersachsen, Germany|GSK Investigational Site, Hannover, Niedersachsen, Germany|GSK Investigational Site, Rotenburg (Wuemme), Niedersachsen, Germany|GSK Investigational Site, Aachen, Nordrhein-Westfalen, Germany|GSK Investigational Site, Bochum, Nordrhein-Westfalen, Germany|GSK Investigational Site, Bonn, Nordrhein-Westfalen, Germany|GSK Investigational Site, Dortmund, Nordrhein-Westfalen, Germany|GSK Investigational Site, Duesseldorf, Nordrhein-Westfalen, Germany|GSK Investigational Site, Essen, Nordrhein-Westfalen, Germany|GSK Investigational Site, Herne, Nordrhein-Westfalen, Germany|GSK Investigational Site, Koeln, Nordrhein-Westfalen, Germany|GSK Investigational Site, Leverkusen, Nordrhein-Westfalen, Germany|GSK Investigational Site, Muenster, Nordrhein-Westfalen, Germany|GSK Investigational Site, Oberhausen, Nordrhein-Westfalen, Germany|GSK Investigational Site, Siegen, Nordrhein-Westfalen, Germany|GSK Investigational Site, Mainz, Rheinland-Pfalz, Germany|GSK Investigational Site, Halle, Sachsen-Anhalt, Germany|GSK Investigational Site, Magdeburg, Sachsen-Anhalt, Germany|GSK Investigational Site, Chemnitz, Sachsen, Germany|GSK Investigational Site, Leipzig, Sachsen, Germany|GSK Investigational Site, Berlin, Germany|GSK Investigational Site, Berlin, Germany|GSK Investigational Site, Berlin, Germany|GSK Investigational Site, Berlin, Germany|GSK Investigational Site, Hamburg, Germany|GSK Investigational Site, Hamburg, Germany|GSK Investigational Site, Athens, Greece|GSK Investigational Site, Athens, Greece|GSK Investigational Site, Rio, Patras, Greece|GSK Investigational Site, Thessaloniki, Greece|GSK Investigational Site, Bangalore, India|GSK Investigational Site, Chennai, India|GSK Investigational Site, Hyderabad, India|GSK Investigational Site, Mumbai, India|GSK Investigational Site, Mumbai, India|GSK Investigational Site, Mumbai, India|GSK Investigational Site, Haifa, Israel|GSK Investigational Site, Haifa, Israel|GSK Investigational Site, Jerusalem, Israel|GSK Investigational Site, Nazareth, Israel|GSK Investigational Site, Petach Tikva, Israel|GSK Investigational Site, Rehovot, Israel|GSK Investigational Site, Safed, Israel|GSK Investigational Site, Tel Aviv, Israel|GSK Investigational Site, Catanzaro, Calabria, Italy|GSK Investigational Site, Avellino, Campania, Italy|GSK Investigational Site, Bologna, Emilia-Romagna, Italy|GSK Investigational Site, Roma, Lazio, Italy|GSK Investigational Site, Genova, Liguria, Italy|GSK Investigational Site, Brescia, Lombardia, Italy|GSK Investigational Site, Milano, Lombardia, Italy|GSK Investigational Site, Milano, Lombardia, Italy|GSK Investigational Site, Bari, Puglia, Italy|GSK Investigational Site, San Giovanni Rotondo (FG), Puglia, Italy|GSK Investigational Site, Palermo, Sicilia, Italy|GSK Investigational Site, Busan, Korea, Republic of|GSK Investigational Site, Daegu, Korea, Republic of|GSK Investigational Site, Pusan, Korea, Republic of|GSK Investigational Site, Seoul, Korea, Republic of|GSK Investigational Site, Seoul, Korea, Republic of|GSK Investigational Site, Lahore, Pakistan|GSK Investigational Site, Lahore, Pakistan|GSK Investigational Site, Chorzow, Poland|GSK Investigational Site, Kielce, Poland|GSK Investigational Site, Raciborz, Poland|GSK Investigational Site, Szczecin, Poland|GSK Investigational Site, Warszawa, Poland|GSK Investigational Site, Wroclaw, Poland|GSK Investigational Site, Ponce, Puerto Rico|GSK Investigational Site, San Juan, Puerto Rico|GSK Investigational Site, Bucharest, Romania|GSK Investigational Site, Constanta, Romania|GSK Investigational Site, Moscow, Russian Federation|GSK Investigational Site, Saint-Petersburg, Russian Federation|GSK Investigational Site, Samara, Russian Federation|GSK Investigational Site, St.Peterburg, Russian Federation|GSK Investigational Site, Bratislava, Slovakia|GSK Investigational Site, Bratislava, Slovakia|GSK Investigational Site, Kosice, Slovakia|GSK Investigational Site, Martin, Slovakia|GSK Investigational Site, Alicante, Spain|GSK Investigational Site, Barcelona, Spain|GSK Investigational Site, Barcelona, Spain|GSK Investigational Site, La Coruña, Spain|GSK Investigational Site, Madrid, Spain|GSK Investigational Site, Madrid, Spain|GSK Investigational Site, Madrid, Spain|GSK Investigational Site, Málaga, Spain|GSK Investigational Site, Palma de Mallorca, Spain|GSK Investigational Site, Pontevedra, Spain|GSK Investigational Site, Sabadell (Barcelona), Spain|GSK Investigational Site, San Sebastián, Spain|GSK Investigational Site, Sevilla, Spain|GSK Investigational Site, Valencia, Spain|GSK Investigational Site, Valencia, Spain|GSK Investigational Site, Changhua, Taiwan|GSK Investigational Site, Chia-Yi Hsien, Taiwan|GSK Investigational Site, Kaohsiung, Taiwan|GSK Investigational Site, Taichung, Taiwan|GSK Investigational Site, Taipei, Taiwan|GSK Investigational Site, Taiyuan Hsien, Taiwan|GSK Investigational Site, Donetsk, Ukraine|GSK Investigational Site, Kiev, Ukraine|GSK Investigational Site, Vinnitsa, Ukraine
URL: https://ClinicalTrials.gov/show/NCT00529568
Study 54:
Title: Eltrombopag To Initiate And Maintain Interferon Antiviral Treatment To Subjects With Hepatitis C Related Liver Disease
Status: Completed
Study Results: Has Results
Conditions: Hepatitis C, Chronic
Interventions: Drug: eltrombopag|Drug: placebo
Locations: GSK Investigational Site, Birmingham, Alabama, United States|GSK Investigational Site, Tucson, Arizona, United States|GSK Investigational Site, Little Rock, Arkansas, United States|GSK Investigational Site, La Jolla, California, United States|GSK Investigational Site, Los Angeles, California, United States|GSK Investigational Site, Los Angeles, California, United States|GSK Investigational Site, San Clemente, California, United States|GSK Investigational Site, Aurora, Colorado, United States|GSK Investigational Site, New Haven, Connecticut, United States|GSK Investigational Site, Washington, District of Columbia, United States|GSK Investigational Site, Washington, District of Columbia, United States|GSK Investigational Site, Bradenton, Florida, United States|GSK Investigational Site, Gainsville, Florida, United States|GSK Investigational Site, Miami, Florida, United States|GSK Investigational Site, Orlando, Florida, United States|GSK Investigational Site, Atlanta, Georgia, United States|GSK Investigational Site, Honolulu, Hawaii, United States|GSK Investigational Site, Louisville, Kentucky, United States|GSK Investigational Site, Baltimore, Maryland, United States|GSK Investigational Site, Burlington, Massachusetts, United States|GSK Investigational Site, Worcester, Massachusetts, United States|GSK Investigational Site, Ann Arbor, Michigan, United States|GSK Investigational Site, Detroit, Michigan, United States|GSK Investigational Site, Jackson, Mississippi, United States|GSK Investigational Site, St. Louis, Missouri, United States|GSK Investigational Site, Bronx, New York, United States|GSK Investigational Site, Manhasset, New York, United States|GSK Investigational Site, New York, New York, United States|GSK Investigational Site, Rochester, New York, United States|GSK Investigational Site, Syracuse, New York, United States|GSK Investigational Site, Valhalla, New York, United States|GSK Investigational Site, Asheville, North Carolina, United States|GSK Investigational Site, Durham, North Carolina, United States|GSK Investigational Site, Tulsa, Oklahoma, United States|GSK Investigational Site, Portland, Oregon, United States|GSK Investigational Site, Hershey, Pennsylvania, United States|GSK Investigational Site, Philadelphia, Pennsylvania, United States|GSK Investigational Site, Jackson, Tennessee, United States|GSK Investigational Site, Nashville, Tennessee, United States|GSK Investigational Site, Nashville, Tennessee, United States|GSK Investigational Site, Nashville, Tennessee, United States|GSK Investigational Site, Galveston, Texas, United States|GSK Investigational Site, Houston, Texas, United States|GSK Investigational Site, San Antonio, Texas, United States|GSK Investigational Site, Charlottesville, Virginia, United States|GSK Investigational Site, Fairfax, Virginia, United States|GSK Investigational Site, Richmond, Virginia, United States|GSK Investigational Site, Garran, Australian Capital Territory, Australia|GSK Investigational Site, Camperdown, New South Wales, Australia|GSK Investigational Site, Randwick, New South Wales, Australia|GSK Investigational Site, Fitzroy, Victoria, Australia|GSK Investigational Site, Heidelberg, Victoria, Australia|GSK Investigational Site, Melbourne, Victoria, Australia|GSK Investigational Site, Nedlands, Western Australia, Australia|GSK Investigational Site, Bruxelles, Belgium|GSK Investigational Site, Edegem, Belgium|GSK Investigational Site, Gent, Belgium|GSK Investigational Site, Leuven, Belgium|GSK Investigational Site, Campinas, São Paulo, Brazil|GSK Investigational Site, São Paulo, Brazil|GSK Investigational Site, Calgary, Alberta, Canada|GSK Investigational Site, Victoria, British Columbia, Canada|GSK Investigational Site, Winnipeg, Manitoba, Canada|GSK Investigational Site, Barrie, Ontario, Canada|GSK Investigational Site, Hamilton, Ontario, Canada|GSK Investigational Site, Hamilton, Ontario, Canada|GSK Investigational Site, Ottawa, Ontario, Canada|GSK Investigational Site, Ottawa, Ontario, Canada|GSK Investigational Site, Toronto, Ontario, Canada|GSK Investigational Site, Toronto, Ontario, Canada|GSK Investigational Site, Toronto, Ontario, Canada|GSK Investigational Site, Toronto, Ontario, Canada|GSK Investigational Site, Montreal, Quebec, Canada|GSK Investigational Site, Montreal, Quebec, Canada|GSK Investigational Site, Praha 4, Czech Republic|GSK Investigational Site, Praha 6, Czech Republic|GSK Investigational Site, Besançon, France|GSK Investigational Site, Dijon cedex, France|GSK Investigational Site, Marseille, France|GSK Investigational Site, Montpellier, France|GSK Investigational Site, Nice, France|GSK Investigational Site, Pessac Cedex, France|GSK Investigational Site, Strasbourg, France|GSK Investigational Site, Toulouse cedex 9, France|GSK Investigational Site, Toulouse, France|GSK Investigational Site, Freiburg, Baden-Wuerttemberg, Germany|GSK Investigational Site, Heidelberg, Baden-Wuerttemberg, Germany|GSK Investigational Site, Stuttgart, Baden-Wuerttemberg, Germany|GSK Investigational Site, Ulm, Baden-Wuerttemberg, Germany|GSK Investigational Site, Deggendorf, Bayern, Germany|GSK Investigational Site, Hof/Saale, Bayern, Germany|GSK Investigational Site, Muenchen, Bayern, Germany|GSK Investigational Site, Regensburg, Bayern, Germany|GSK Investigational Site, Wuerzburg, Bayern, Germany|GSK Investigational Site, Beeskow, Brandenburg, Germany|GSK Investigational Site, Frankfurt, Hessen, Germany|GSK Investigational Site, Kassel, Hessen, Germany|GSK Investigational Site, Goettingen, Niedersachsen, Germany|GSK Investigational Site, Hannover, Niedersachsen, Germany|GSK Investigational Site, Hannover, Niedersachsen, Germany|GSK Investigational Site, Aachen, Nordrhein-Westfalen, Germany|GSK Investigational Site, Bochum, Nordrhein-Westfalen, Germany|GSK Investigational Site, Bonn, Nordrhein-Westfalen, Germany|GSK Investigational Site, Dortmund, Nordrhein-Westfalen, Germany|GSK Investigational Site, Duesseldorf, Nordrhein-Westfalen, Germany|GSK Investigational Site, Essen, Nordrhein-Westfalen, Germany|GSK Investigational Site, Herne, Nordrhein-Westfalen, Germany|GSK Investigational Site, Koeln, Nordrhein-Westfalen, Germany|GSK Investigational Site, Leverkusen, Nordrhein-Westfalen, Germany|GSK Investigational Site, Muenster, Nordrhein-Westfalen, Germany|GSK Investigational Site, Siegen, Nordrhein-Westfalen, Germany|GSK Investigational Site, Mainz, Rheinland-Pfalz, Germany|GSK Investigational Site, Halle, Sachsen-Anhalt, Germany|GSK Investigational Site, Magdeburg, Sachsen-Anhalt, Germany|GSK Investigational Site, Leipzig, Sachsen, Germany|GSK Investigational Site, Leipzig, Sachsen, Germany|GSK Investigational Site, Berlin, Germany|GSK Investigational Site, Berlin, Germany|GSK Investigational Site, Pokfulam, Hong Kong|GSK Investigational Site, Bangalore, India|GSK Investigational Site, Chennai, India|GSK Investigational Site, Mumbai, India|GSK Investigational Site, Haifa, Israel|GSK Investigational Site, Jerusalem, Israel|GSK Investigational Site, Petach Tikva, Israel|GSK Investigational Site, Safed, Israel|GSK Investigational Site, Tel Aviv, Israel|GSK Investigational Site, Catanzaro, Calabria, Italy|GSK Investigational Site, Avellino, Campania, Italy|GSK Investigational Site, Bologna, Emilia-Romagna, Italy|GSK Investigational Site, Roma, Lazio, Italy|GSK Investigational Site, Genova, Liguria, Italy|GSK Investigational Site, Brescia, Lombardia, Italy|GSK Investigational Site, Milano, Lombardia, Italy|GSK Investigational Site, Milano, Lombardia, Italy|GSK Investigational Site, Bari, Puglia, Italy|GSK Investigational Site, San Giovanni Rotondo (FG), Puglia, Italy|GSK Investigational Site, Palermo, Sicilia, Italy|GSK Investigational Site, Busan, Korea, Republic of|GSK Investigational Site, Incheon, Korea, Republic of|GSK Investigational Site, Seoul, Korea, Republic of|GSK Investigational Site, Amsterdam, Netherlands|GSK Investigational Site, Nijmegen, Netherlands|GSK Investigational Site, Rotterdam, Netherlands|GSK Investigational Site, Lahore, Pakistan|GSK Investigational Site, Lahore, Pakistan|GSK Investigational Site, Bydgoszcz, Poland|GSK Investigational Site, Chorzow, Poland|GSK Investigational Site, Kielce, Poland|GSK Investigational Site, Szczecin, Poland|GSK Investigational Site, Wroclaw, Poland|GSK Investigational Site, Ponce, Puerto Rico|GSK Investigational Site, San Juan, Puerto Rico|GSK Investigational Site, Bucharest, Romania|GSK Investigational Site, Constanta, Romania|GSK Investigational Site, Moscow, Russian Federation|GSK Investigational Site, Moscow, Russian Federation|GSK Investigational Site, Mosocow, Russian Federation|GSK Investigational Site, Smolensk, Russian Federation|GSK Investigational Site, Bratislava, Slovakia|GSK Investigational Site, Bratislava, Slovakia|GSK Investigational Site, Kosice, Slovakia|GSK Investigational Site, Martin, Slovakia|GSK Investigational Site, Barcelona, Spain|GSK Investigational Site, Granada, Spain|GSK Investigational Site, L’Hospitalet de Llobregat. Barcelona, Spain|GSK Investigational Site, La Coruña, Spain|GSK Investigational Site, Madrid, Spain|GSK Investigational Site, Madrid, Spain|GSK Investigational Site, Madrid, Spain|GSK Investigational Site, Madrid, Spain|GSK Investigational Site, San Sebastián, Spain|GSK Investigational Site, Sevilla, Spain|GSK Investigational Site, Valencia, Spain|GSK Investigational Site, Kaohsiung, Taiwan|GSK Investigational Site, Taichung, Taiwan|GSK Investigational Site, Tainan, Taiwan|GSK Investigational Site, Taipei, Taiwan|GSK Investigational Site, Bangkok, Thailand|GSK Investigational Site, Bangkok, Thailand|GSK Investigational Site, Chiangmai, Thailand|GSK Investigational Site, Khon Kaen, Thailand|GSK Investigational Site, Songkla, Thailand|GSK Investigational Site, Donetsk, Ukraine|GSK Investigational Site, Kyiv, Ukraine|GSK Investigational Site, Kyiv, Ukraine|GSK Investigational Site, Vinnytsia, Ukraine|GSK Investigational Site, Glasgow, Lanarkshire, United Kingdom|GSK Investigational Site, London, United Kingdom|GSK Investigational Site, London, United Kingdom|GSK Investigational Site, Plymouth, United Kingdom
URL: https://ClinicalTrials.gov/show/NCT00516321
Study 55:
Title: Conversion to Embeda With Rescue Trial
Status: Terminated
Study Results: Has Results
Conditions: Chronic Disease|Pain
Interventions: Drug: morphine sulfate and naltrexone hydrochloride (EMBEDA)
Locations: Adamsville Family Medicine, Adamsville, Alabama, United States|Office of David McLain, Birmingham, Alabama, United States|Monte Sano Clinical Research, LLC, Huntsville, Alabama, United States|Tennessee Valley Pain Consultants Properties, LLC, Huntsville, Alabama, United States|Sunbelt Research Group, LLC, Mobile, Alabama, United States|Office of Vaughn H. Mancha, Jr., PC, Montgomery, Alabama, United States|Dedicated Clinical Research, Goodyear, Arizona, United States|Dedicated Clinical Research, Inc, Phoenix, Arizona, United States|Redpoint Research, Phoenix, Arizona, United States|Anasazi Internal Medicine, PC, Phoenix, Arizona, United States|Cochise Clinical Research, Sierra Vista, Arizona, United States|Premiere Phamaceutical Research, LLC, Tempe, Arizona, United States|Quality of Life Medical and Research Center, LLC, Tucson, Arizona, United States|Ouachita Regional Pain Management, Hot Springs, Arkansas, United States|NEA Baptist Clinic, Jonesboro, Arkansas, United States|Hollis Family Medical Clinic, PLC, Paragould, Arkansas, United States|CSI Clinical Trials, Costa Mesa, California, United States|Global Wellness Medical Corporation, Foothill Ranch, California, United States|Chrishard Medical Group, Inglewood, California, United States|Triwest Research Associates LLC, La Mesa, California, United States|Pacific Coast Pain Management Center, Laguna Hills, California, United States|Valerius Medical Group and Research Center of Greater Long Beach, Inc., Long Beach, California, United States|LA Pain & Wellness Institute, Los Angeles, California, United States|Samaritan Center for Medical Research, Los Gatos, California, United States|Newport Beach Clinical Research Associates, Inc., Newport Beach, California, United States|Bayview Research Group, LLC, Paramount, California, United States|Pasadena Rehabilitation Institute, Pasadena, California, United States|Quality Control Research, Inc., Roseville, California, United States|Northern California Research, Sacramento, California, United States|Quality Control Research, Inc., Sacramento, California, United States|Rancho Santa Fe Medical Group, Inc., San Marcos, California, United States|Probe Clinical Research Corporation, Santa Ana, California, United States|Trinity Clinical Trials, Santa Ana, California, United States|Facility Medical Center, Upland, California, United States|Bayview Research Group, LLC, Valley Village, California, United States|Rocky Mountain Internal Medicine, PC, Aurora, Colorado, United States|Clinicos, LLC, Colorado Springs, Colorado, United States|Saint Luke’s Medical Clinic, LLC, Fort Collins, Colorado, United States|ProHealth Physicians PC, Manchester, Connecticut, United States|Milford Physician Services, PC, Milford, Connecticut, United States|Orthopedic Research Institute, LLC, Boynton Beach, Florida, United States|Florida Research & Testing, LLC, Clearwater, Florida, United States|Omega Research Consultants, LLC, Debary, Florida, United States|Omega Research Consultants, LLC, DeBary, Florida, United States|West Florida Medical Associate, PA, Dunnellon, Florida, United States|International Research Associates, LLC, Hialeah, Florida, United States|Palm Springs Research Institute, Inc, Hialeah, Florida, United States|FPA Clinical Research, LLC, Kissimmee, Florida, United States|Clinical Research of Central Florida, Inc., Lakeland, Florida, United States|NextPhase Clinical Trials, Inc., Miami Beach, Florida, United States|Community Research Foundation, Inc., Miami, Florida, United States|New Horizon Research Center, Inc., Miami, Florida, United States|Harmony Clinical Research, Inc., North Miami Beach, Florida, United States|Office of Laszlo J. Mate, MD, PA, North Palm Beach, Florida, United States|Office of Richard E. Promin, MD, PA, Ocala, Florida, United States|Advent Clinical Research Centers, Inc., Pinellas Park, Florida, United States|Pain Management Strategies, Inc., Pompano Beach, Florida, United States|Sarasota Pain Medicine Research, LLC, Sarasota, Florida, United States|Stedman Clinical Trials, LLC, Tampa, Florida, United States|Clinical Research Center, LLC, Wellington, Florida, United States|Perimeter Institute for Clinical Research, Inc., Atlanta, Georgia, United States|Medical Research and Health Education Foundation, Inc., Columbus, Georgia, United States|Ialum Clinical Research, LLC, Decatur, Georgia, United States|Ialum Clinical Research, LLC, Stone Mountain, Georgia, United States|Herman Clinical Research, LLC, Suwanee, Georgia, United States|Centers for Pain Management, Tifton, Georgia, United States|Chicago Clinical Research Institute Inc., Chicago, Illinois, United States|Creve Coeur Family Practice, Creve Coeur, Illinois, United States|Office of Rebecca Knight, MD, Peoria, Illinois, United States|Josephson Wallack Munshower Neurology P.C., Indianapolis, Indiana, United States|Laporte County Institute for Clinical Research Inc., Michigan City, Indiana, United States|McKinley Research, LLC, Mishawaka, Indiana, United States|Accelovance, Inc., South Bend, Indiana, United States|Des Moines Orthopaedic Surgeons, PC, West Des Moines, Iowa, United States|The Pain Treatment Center of the Bluegrass, Lexington, Kentucky, United States|Healing Options, Louisville, Kentucky, United States|Four Rivers Clinical Research, Inc., Paducah, Kentucky, United States|Lakewood Family Practice, Russell Springs, Kentucky, United States|Diseasebusters, LLC, College Park, Maryland, United States|Office of Steven C. Miller, MD, Pikesville, Maryland, United States|Beacon Clinical Research, LLC, Brockton, Massachusetts, United States|Ronald J. Rapoport, MD, PC, Fall River, Massachusetts, United States|Boston Paincare Center, Inc., Waltham, Massachusetts, United States|Clarkston Medical Group, PC, Clarkston, Michigan, United States|Apex Medical Research, AMR, Inc., Flint, Michigan, United States|East Michigan Medical Associates, Flint, Michigan, United States|PCM Medical Services, PC, Lansing, Michigan, United States|Remedica LLC, Rochester, Michigan, United States|Michigan Lifestyle Change and Health Center, PC, Sterling, Michigan, United States|MAPS Applied Research Center, Inc., Edina, Minnesota, United States|MAPS Applied Research Center, Inc., Shakopee, Minnesota, United States|Anesthesia and Pain Control Services, Biloxi, Mississippi, United States|CRC of Jackson, LLC, Jackson, Mississippi, United States|Midsouth Anesthesia Consultants, PLLC, Southhaven, Mississippi, United States|Patterson Medical Clinic, Inc., Florissant, Missouri, United States|Quality Clinical Research Inc., Florissant, Missouri, United States|Primary Care Medicine, PC, Jefferson City, Missouri, United States|The Reiter Foundation, Inc., Anaconda, Montana, United States|Medical Pain Relief Clinic, Omaha, Nebraska, United States|Omaha Clinical Research, PC, Omaha, Nebraska, United States|Atco Medical Associates, PC, Atco, New Jersey, United States|Office of John V. Bernard, MD, Belvidere, New Jersey, United States|Central Jersey Medical Research Center, Inc., Elizabeth, New Jersey, United States|Center for Pain Management, Hackensack, New Jersey, United States|Advocare Heights Primary Care, Haddon Heights, New Jersey, United States|NJ Heart, LLC, Linden, New Jersey, United States|Spine and Pain Centers, PA, Shrewsbury, New Jersey, United States|Premier Research, Inc., Trenton, New Jersey, United States|Adirondack Medical Research Center, Glens Falls, New York, United States|Long Island Gastrointestinal Research Group LLP, Great Neck, New York, United States|Drug Trials America, Inc., Hartsdale, New York, United States|Office of Roger Kasendorf, DO, Long Beach, New York, United States|Family Health Medical Services PLLC, Mayville, New York, United States|New York Spine & Wellness Center, North Syracuse, New York, United States|North American Partners in Pain Management, LLP, Valley Stream, New York, United States|Carolina Clinical Research and Consulting, LLC, Asheboro, North Carolina, United States|Carolina Clinical Research and Consulting, LLC, Asheboro, North Carolina, United States|Joint and Muscle Research Institute, Inc., Charlotte, North Carolina, United States|Catawba Valley Internal Medicine, Hickory, North Carolina, United States|Profen Research Network at ECMA, Jacksonville, North Carolina, United States|The Center For Clinical Research, LLC, Winston-Salem, North Carolina, United States|Medical Frontiers, LLC, Carlisle, Ohio, United States|Valley Medical Research, Centerville, Ohio, United States|Hightop Medical Research Center, Cincinnati, Ohio, United States|Sentral Clinical Research Services, Cincinnati, Ohio, United States|Delaware Smith Clinic Research, Delaware, Ohio, United States|Medical Frontiers, LLC, Franklin, Ohio, United States|Jeffrey J. Haggenjos, DO, Inc., New Lexington, Ohio, United States|Whole Family Medical Care LLC, Perrysburg, Ohio, United States|Office of Jocelyn F. Shimek, DO, Salem, Ohio, United States|Office of James Lassiter, Tiffin, Ohio, United States|Health Research Institute, LLC, Oklahoma City, Oklahoma, United States|Office of Siavash Nael, MD, Inc., Oklahoma City, Oklahoma, United States|Associates of Medicine/John D. Williams, MD, PLLC, Stillwater, Oklahoma, United States|Portland Rheumatology Clinic, LLC, Lake Oswego, Oregon, United States|Office of Joseph E. Yankee, DO, PC, Milwaukie, Oregon, United States|Pennsylvania Pain Specialists, PC, Allentown, Pennsylvania, United States|Ware Medical Associates, PC, Aston, Pennsylvania, United States|Altoona Center for Clinical Research, PC, Duncansville, Pennsylvania, United States|Kandra, Fierer, Kuskin Associates, Ltd., Harrisburg, Pennsylvania, United States|Onuorah Umeh, M.D. P.C, Philadelphia, Pennsylvania, United States|Founders Research Corporation, Philadelphia, Pennsylvania, United States|Progressive Pain Solutions, LLC, Wind Gap, Pennsylvania, United States|Hartwell Research Group, LLC, Anderson, South Carolina, United States|Low Country Rheumatology, PA, Charleston, South Carolina, United States|Pharmacorp Clinical Trials, Inc., Charleston, South Carolina, United States|Internal Medicine of Greer Research LLC, Greer, South Carolina, United States|Clinical Research Authority, LLC, Murrells Inlet, South Carolina, United States|Trident Institute of Medical Research, LLC, North Charleston, South Carolina, United States|Low Country Pain Center, LLC, Orangeburg, South Carolina, United States|Brown Clinic, PLLP, Watertown, South Dakota, United States|Chattanooga Medical Research, LLC, Chattanooga, Tennessee, United States|Comprehensive Pain Specialists, Hendersonville, Tennessee, United States|Corsicana Medical Research, PLLC, Corsicana, Texas, United States|DCT – Genesis Neighborhood Research, LLC, Dallas, Texas, United States|Southwest Urgent Care Center, El Paso, Texas, United States|Westbury Medical Clinic, Houston, Texas, United States|Accurate Clinical Research, Inc., Houston, Texas, United States|Medstar Clinical Research, Houston, Texas, United States|Texas Medical Research Associates, LLC, San Antonio, Texas, United States|Hillcrest Family Health Center, Division of Clinical Research, Waco, Texas, United States|Hillcrest Family Health Center, Waco, Texas, United States|Progressive Clinical Research, LLC, Bountiful, Utah, United States|Sentara Medical Group, NDC Medical Center, Norfolk, Virginia, United States|Washington Center for Pain Management PLLC, Edmonds, Washington, United States|Pain Care, PLLC, Huntington, West Virginia, United States
URL: https://ClinicalTrials.gov/show/NCT01179191
Study 56:
Title: JBT-101 in Systemic Lupus Erythematosus (SLE)
Status: Recruiting
Study Results: No Results Available
Conditions: Systemic Lupus Erythematosus|SLE|Lupus
Interventions: Drug: JBT-101|Drug: Placebo
Locations: University of California San Diego School of Medicine: Division of Rheumatology, Allergy and Immunology, La Jolla, California, United States|UCLA Medical Center: Division of Rheumatology, Los Angeles, California, United States|University of California San Francisco School of Medicine: Lupus Clinic and Rheumatology Clinical Research Center, San Francisco, California, United States|Yale University, New Haven, Connecticut, United States|Bronx-Lebanon Hospital Center: Division of Rheumatology, Bronx, New York, United States|Feinstein Institute for Medical Research: Center for Autoimmune and Musculoskeletal Diseases, Manhasset, New York, United States|New York University Langone Medical Center: Department of Medicine, Division of Rheumatology, New York, New York, United States|Columbia University Medical Center: Department of Medicine, Division of Rheumatology, New York, New York, United States|Duke University, Durham, North Carolina, United States|MetroHealth Medical Center, Cleveland, Ohio, United States|Penn State MS Hershey Medical Center, Hershey, Pennsylvania, United States|University of Pennsylvania, Philadelphia, Pennsylvania, United States|Temple University, Philadelphia, Pennsylvania, United States|University of Pittsburgh Medical Center: Division of Rheumatology and Clinical Immunology, Pittsburgh, Pennsylvania, United States|Medical University of South Carolina, Charleston, South Carolina, United States
URL: https://ClinicalTrials.gov/show/NCT03093402
Study 57:
Title: Maintenance Chemotherapy With or Without Local Consolidative Therapy in Treating Patients With Stage IV Non-small Cell Lung Cancer
Status: Recruiting
Study Results: No Results Available
Conditions: Recurrent Non-Small Cell Lung Carcinoma|Stage IV Non-Small Cell Lung Cancer
Interventions: Radiation: 3-Dimensional Conformal Radiation Therapy (3D-CRT)|Drug: Docetaxel|Drug: Gemcitabine|Radiation: Intensity-Modulated Radiation Therapy (IMRT)|Drug: Pemetrexed Disodium|Radiation: Stereotactic Body Radiation Therapy (SBRT)|Drug: Erlotinib Hydrochloride|Drug: Pembrolizumab
Locations: Arizona Breast Cancer Specialists-Gilbert, Gilbert, Arizona, United States|Arizona Center for Cancer Care-Peoria, Peoria, Arizona, United States|Mayo Clinic Hospital, Phoenix, Arizona, United States|Arizona Breast Cancer Specialists-Scottsdale, Scottsdale, Arizona, United States|Mayo Clinic in Arizona, Scottsdale, Arizona, United States|University of Arizona Cancer Center-Orange Grove Campus, Tucson, Arizona, United States|The University of Arizona Medical Center-University Campus, Tucson, Arizona, United States|University of Arkansas for Medical Sciences, Little Rock, Arkansas, United States|Alta Bates Summit Medical Center-Herrick Campus, Berkeley, California, United States|City of Hope Comprehensive Cancer Center, Duarte, California, United States|UC San Diego Moores Cancer Center, La Jolla, California, United States|Los Angeles County-USC Medical Center, Los Angeles, California, United States|USC / Norris Comprehensive Cancer Center, Los Angeles, California, United States|Saint Joseph Hospital – Orange, Orange, California, United States|The Permanente Medical Group-Roseville Radiation Oncology, Roseville, California, United States|Sutter Medical Center Sacramento, Sacramento, California, United States|University of California Davis Comprehensive Cancer Center, Sacramento, California, United States|Saint Helena Hospital, Saint Helena, California, United States|City of Hope South Pasadena, South Pasadena, California, United States|Kaiser Permanente Cancer Treatment Center, South San Francisco, California, United States|Gene Upshaw Memorial Tahoe Forest Cancer Center, Truckee, California, United States|Penrose-Saint Francis Healthcare, Colorado Springs, Colorado, United States|UCHealth Memorial Hospital Central, Colorado Springs, Colorado, United States|Poudre Valley Hospital, Fort Collins, Colorado, United States|Yale University, New Haven, Connecticut, United States|Helen F Graham Cancer Center, Newark, Delaware, United States|Beebe Health Campus, Rehoboth Beach, Delaware, United States|University of Florida Health Science Center – Gainesville, Gainesville, Florida, United States|Mayo Clinic in Florida, Jacksonville, Florida, United States|Tallahassee Memorial HealthCare, Tallahassee, Florida, United States|Moffitt Cancer Center, Tampa, Florida, United States|Cleveland Clinic-Weston, Weston, Florida, United States|Grady Health System, Atlanta, Georgia, United States|Emory University Hospital Midtown, Atlanta, Georgia, United States|Emory University Hospital/Winship Cancer Institute, Atlanta, Georgia, United States|Emory Saint Joseph’s Hospital, Atlanta, Georgia, United States|Lewis Cancer and Research Pavilion at Saint Joseph’s/Candler, Savannah, Georgia, United States|Queen’s Medical Center, Honolulu, Hawaii, United States|The Cancer Center of Hawaii-Liliha, Honolulu, Hawaii, United States|Northwestern University, Chicago, Illinois, United States|Decatur Memorial Hospital, Decatur, Illinois, United States|Crossroads Cancer Center, Effingham, Illinois, United States|Condell Memorial Hospital, Libertyville, Illinois, United States|Loyola University Medical Center, Maywood, Illinois, United States|Methodist Medical Center of Illinois, Peoria, Illinois, United States|OSF Saint Francis Medical Center, Peoria, Illinois, United States|Memorial Medical Center, Springfield, Illinois, United States|Southwest Illinois Health Services LLP, Swansea, Illinois, United States|Carle Cancer Center, Urbana, Illinois, United States|Parkview Hospital Randallia, Fort Wayne, Indiana, United States|Parkview Regional Medical Center, Fort Wayne, Indiana, United States|Goshen Center for Cancer Care, Goshen, Indiana, United States|Community Cancer Center East, Indianapolis, Indiana, United States|Community Cancer Center South, Indianapolis, Indiana, United States|Franciscan Health Indianapolis, Indianapolis, Indiana, United States|Community Cancer Center North, Indianapolis, Indiana, United States|Franciscan Health Mooresville, Mooresville, Indiana, United States|Iowa Methodist Medical Center, Des Moines, Iowa, United States|University of Kansas Cancer Center, Kansas City, Kansas, United States|Lawrence Memorial Hospital, Lawrence, Kansas, United States|University of Kansas Cancer Center-Overland Park, Overland Park, Kansas, United States|Via Christi Regional Medical Center, Wichita, Kansas, United States|The James Graham Brown Cancer Center at University of Louisville, Louisville, Kentucky, United States|LSU Health Baton Rouge-North Clinic, Baton Rouge, Louisiana, United States|Louisiana Hematology Oncology Associates LLC, Baton Rouge, Louisiana, United States|Mary Bird Perkins Cancer Center, Baton Rouge, Louisiana, United States|Our Lady of the Lake Physicians Group – Medical Oncology, Baton Rouge, Louisiana, United States|University of Maryland/Greenebaum Cancer Center, Baltimore, Maryland, United States|Central Maryland Radiation Oncology in Howard County, Columbia, Maryland, United States|Tate Cancer Center, Glen Burnie, Maryland, United States|Peninsula Regional Medical Center, Salisbury, Maryland, United States|Boston Medical Center, Boston, Massachusetts, United States|Lahey Hospital and Medical Center, Burlington, Massachusetts, United States|McLaren Cancer Institute-Clarkston, Clarkston, Michigan, United States|Henry Ford Macomb Hospital-Clinton Township, Clinton Township, Michigan, United States|Wayne State University/Karmanos Cancer Institute, Detroit, Michigan, United States|Henry Ford Hospital, Detroit, Michigan, United States|Weisberg Cancer Treatment Center, Farmington Hills, Michigan, United States|McLaren Cancer Institute-Flint, Flint, Michigan, United States|West Michigan Cancer Center, Kalamazoo, Michigan, United States|McLaren Cancer Institute-Macomb, Mount Clemens, Michigan, United States|McLaren Cancer Institute-Northern Michigan, Petoskey, Michigan, United States|McLaren-Port Huron, Port Huron, Michigan, United States|Sanford Joe Lueken Cancer Center, Bemidji, Minnesota, United States|Saint Luke’s Hospital of Duluth, Duluth, Minnesota, United States|Regions Hospital, Saint Paul, Minnesota, United States|Kansas City Veterans Affairs Medical Center, Kansas City, Missouri, United States|Washington University School of Medicine, Saint Louis, Missouri, United States|Missouri Baptist Medical Center, Saint Louis, Missouri, United States|Mercy Hospital Saint Louis, Saint Louis, Missouri, United States|Benefis Healthcare- Sletten Cancer Institute, Great Falls, Montana, United States|Kalispell Regional Medical Center, Kalispell, Montana, United States|Alegent Health Bergan Mercy Medical Center, Omaha, Nebraska, United States|Renown Regional Medical Center, Reno, Nevada, United States|Wentworth-Douglass Hospital, Dover, New Hampshire, United States|Virtua Memorial, Mount Holly, New Jersey, United States|Community Medical Center, Toms River, New Jersey, United States|Virtua Voorhees, Voorhees, New Jersey, United States|Lovelace Medical Center-Saint Joseph Square, Albuquerque, New Mexico, United States|University of New Mexico Cancer Center, Albuquerque, New Mexico, United States|Lovelace Radiation Oncology, Albuquerque, New Mexico, United States|Christus Saint Vincent Regional Cancer Center, Santa Fe, New Mexico, United States|Montefiore Medical Center-Einstein Campus, Bronx, New York, United States|Montefiore Medical Center – Moses Campus, Bronx, New York, United States|Mount Sinai Hospital, New York, New York, United States|University of Rochester, Rochester, New York, United States|Stony Brook University Medical Center, Stony Brook, New York, United States|Dickstein Cancer Treatment Center, White Plains, New York, United States|Duke University Medical Center, Durham, North Carolina, United States|CarolinaEast Medical Center, New Bern, North Carolina, United States|NHRMC Radiation Oncology – Supply, Supply, North Carolina, United States|New Hanover Regional Medical Center/Zimmer Cancer Center, Wilmington, North Carolina, United States|NHRMC Radiation Oncology – 16th Street, Wilmington, North Carolina, United States|Sanford Bismarck Medical Center, Bismarck, North Dakota, United States|Roger Maris Cancer Center, Fargo, North Dakota, United States|Cleveland Clinic Akron General, Akron, Ohio, United States|University of Cincinnati/Barrett Cancer Center, Cincinnati, Ohio, United States|Case Western Reserve University, Cleveland, Ohio, United States|Cleveland Clinic Cancer Center/Fairview Hospital, Cleveland, Ohio, United States|Cleveland Clinic Foundation, Cleveland, Ohio, United States|Ohio State University Comprehensive Cancer Center, Columbus, Ohio, United States|The Mark H Zangmeister Center, Columbus, Ohio, United States|Cleveland Clinic Cancer Center Mansfield, Mansfield, Ohio, United States|Hillcrest Hospital Cancer Center, Mayfield Heights, Ohio, United States|North Coast Cancer Care, Sandusky, Ohio, United States|University Pointe, West Chester, Ohio, United States|University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma, United States|Mercy Hospital Oklahoma City, Oklahoma City, Oklahoma, United States|Good Samaritan Hospital, Corvallis, Oregon, United States|Legacy Mount Hood Medical Center, Gresham, Oregon, United States|Legacy Good Samaritan Hospital and Medical Center, Portland, Oregon, United States|Providence Portland Medical Center, Portland, Oregon, United States|Providence Saint Vincent Medical Center, Portland, Oregon, United States|Bryn Mawr Hospital, Bryn Mawr, Pennsylvania, United States|Geisinger Medical Center, Danville, Pennsylvania, United States|Delaware County Memorial Hospital, Drexel Hill, Pennsylvania, United States|UPMC Pinnacle Cancer Center/Community Osteopathic Campus, Harrisburg, Pennsylvania, United States|Geisinger Medical Oncology-Lewisburg, Lewisburg, Pennsylvania, United States|Paoli Memorial Hospital, Paoli, Pennsylvania, United States|Thomas Jefferson University Hospital, Philadelphia, Pennsylvania, United States|Reading Hospital, West Reading, Pennsylvania, United States|Geisinger Wyoming Valley/Henry Cancer Center, Wilkes-Barre, Pennsylvania, United States|Abington Memorial Hospital-Asplundh Cancer Pavilion, Willow Grove, Pennsylvania, United States|Lankenau Medical Center, Wynnewood, Pennsylvania, United States|Greenville Health System Cancer Institute-Faris, Greenville, South Carolina, United States|Greenville Health System Cancer Institute-Eastside, Greenville, South Carolina, United States|Self Regional Healthcare, Greenwood, South Carolina, United States|Greenville Health System Cancer Institute-Greer, Greer, South Carolina, United States|Gibbs Cancer Center-Pelham, Greer, South Carolina, United States|The Radiation Oncology Center-Hilton Head/Bluffton, Hilton Head Island, South Carolina, United States|Greenville Health System Cancer Institute-Seneca, Seneca, South Carolina, United States|Spartanburg Medical Center, Spartanburg, South Carolina, United States|Greenville Health System Cancer Institute-Spartanburg, Spartanburg, South Carolina, United States|Sanford USD Medical Center – Sioux Falls, Sioux Falls, South Dakota, United States|Baptist Memorial Hospital and Cancer Center-Memphis, Memphis, Tennessee, United States|UT Southwestern/Simmons Cancer Center-Dallas, Dallas, Texas, United States|University of Texas Medical Branch, Galveston, Texas, United States|M D Anderson Cancer Center, Houston, Texas, United States|University of Texas Health Science Center at San Antonio, San Antonio, Texas, United States|West Virginia University Healthcare, Morgantown, West Virginia, United States|Aurora Cancer Care-Southern Lakes VLCC, Burlington, Wisconsin, United States|Aurora Health Center-Fond du Lac, Fond Du Lac, Wisconsin, United States|Aurora Health Care Germantown Health Center, Germantown, Wisconsin, United States|Aurora Cancer Care-Grafton, Grafton, Wisconsin, United States|Saint Vincent Hospital Cancer Center Green Bay, Green Bay, Wisconsin, United States|Saint Vincent Hospital Cancer Center at Saint Mary’s, Green Bay, Wisconsin, United States|Aurora BayCare Medical Center, Green Bay, Wisconsin, United States|Aurora Cancer Care-Kenosha South, Kenosha, Wisconsin, United States|Gundersen Lutheran Medical Center, La Crosse, Wisconsin, United States|Aurora Bay Area Medical Group-Marinette, Marinette, Wisconsin, United States|Aurora Cancer Care-Milwaukee, Milwaukee, Wisconsin, United States|Aurora Saint Luke’s Medical Center, Milwaukee, Wisconsin, United States|Aurora Sinai Medical Center, Milwaukee, Wisconsin, United States|Vince Lombardi Cancer Clinic – Oshkosh, Oshkosh, Wisconsin, United States|Aurora Cancer Care-Racine, Racine, Wisconsin, United States|Vince Lombardi Cancer Clinic-Sheboygan, Sheboygan, Wisconsin, United States|Marshfield Clinic Stevens Point Center, Stevens Point, Wisconsin, United States|Aurora Medical Center in Summit, Summit, Wisconsin, United States|Vince Lombardi Cancer Clinic-Two Rivers, Two Rivers, Wisconsin, United States|Aurora Cancer Care-Milwaukee West, Wauwatosa, Wisconsin, United States|Aurora West Allis Medical Center, West Allis, Wisconsin, United States|London Regional Cancer Program, London, Ontario, Canada|Ottawa Hospital and Cancer Center-General Campus, Ottawa, Ontario, Canada|King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia
URL: https://ClinicalTrials.gov/show/NCT03137771


